Pathology|20 Feb 2023
Spore trapping: what is it, and why is it important for agriculture?
At BioScout, we automate airborne disease tracking in agricultural systems. What does this mean, and why have we undertaken such a task?
Lucy Horne
10 Min Read
BioScout early prototype (left) and DPIRD passive spore trap.
At BioScout, we automate airborne disease tracking in agricultural systems. What does this mean, and why have we undertaken such a task? For this question to be answered, let’s talk about airborne disease tracking and why it matters.
Crops are constantly threatened by various plant pathogens, many of which are spread through the air. When crops are under attack from nasty fungal pathogens such as wheat stripe rust, different disease management strategies are employed for effective control, particularly fungicides. The effectiveness of a fungicide is all about timing. Time your application wrong, and you'll suffer heavy losses from disease. But get your timing right, and a single application could be enough to protect your crop at those vital growth stages. Proper timing also has huge impacts on boosting environmental sustainability and limiting fungicide resistance. So, how can we figure out optimal fungicide timing? Well, the best way to do this is through spore trapping (what?!)
So, what is spore trapping? Spore trapping is as it sounds - the goal is to trap spores carried in the air, mainly for disease surveillance and monitoring. Spore trapping falls under the broader scientific field of aerobiology. Aerobiology is the study of airborne biological particles - ‘life in the air’ - and the processes influencing their transmission and deposition. So, ‘life in the air’ (also coined the ‘air spora’ by Gregory in 1952) includes microorganisms like pollen, bacteria, viruses, protozoa, and what we care about the most - fungal spores. Monitoring these fungal spores that cause disease in plants at an early stage is critical because once plants are symptomatic, the optimal time to spray is gone. But how exactly are these spores trapped?
Passive Traps
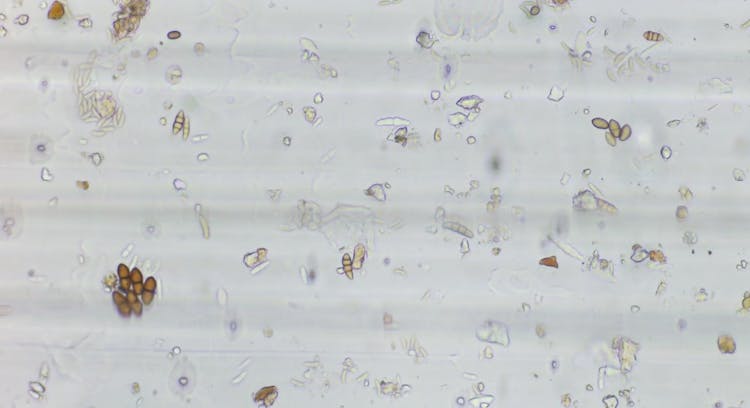
The main trap types used in aerobiology to collect spores are either passive or active. Essentially, when air gets actively sucked into a device, in that case, it is an active trap. If there is no suction mechanism, then it is a passive trap. Passive traps collect spores deposited onto a sticky surface (i.e. tape, glycerine or petroleum jelly on a microscope slide). For example, a basic passive trap can be a microscope slide coated with petroleum jelly attached to a structure for support. They are cheap to purchase or build (you can make one at home, it’s that easy) and directly mounted on a microscope for analysis. However, this method only gives you a raw spore count, and calculating the concentration of spores in the air (i.e. the number of spores per m³ of air) is not possible. This is a problem because knowing airborne spore concentrations is extremely important for disease management.
Passive Spore Sampler. Source: Fig. 1. J. L Blackall., et al.
Active/Volumetric Traps
On the other hand, active traps sample the air and collect spores through a mechanism such as a rotor or a pump. They are also called volumetric traps because the volume of air sampled is known (e.g. 10 litres per minute), and airborne spore concentrations are calculated. Active samplers are broadly classified into the following groups: impaction samplers, cyclone samplers, ionic samplers and virtual impactors. For impaction samplers, as the name implies, spores impact onto sticky surfaces like tape when collected. Cyclone samplers collect spores directly into a small tube, whereas ionic samplers use electron microscope electrodes and a strong electric field to collect spores. In contrast, virtual impactor samplers capture spores without direct impaction by separating smaller and larger particles into two air currents. Within these categories, there are a variety of different devices and brands available. See image 1 below for more context!
One of the first volumetric traps was invented in 1871 when P. Miguel conducted a longitudinal study sampling air in Paris. He built a volumetric trap using a water-operated pump which produced a suction of 20 litres of air per hour through an aperture to impact spores on a glycerine-coated glass slide. Today, the kind of spore trapping we use was pioneered by Jim Hirst in 1952. Hirst realised that spore traps needed reliable suction to sample the air because suction traps had a few key issues. The cascade impactor, a four-stage suction trap, was generally used as the standard for calibrating other traps. Yet, the slides on these traps quickly became overloaded with airborne particles, making it challenging to identify the different spores. So, Hirst built a trap with a mechanism to continuously move a sticky slide across an orifice throughout the day. This mechanism also meant that the time of spore deposition could be calculated and correlated to environmental conditions, paving the way for a deeper understanding of plant disease epidemiology. Brilliant! Hirst’s trap was further improved and then produced by Burkard Manufacturing Co. Ltd in the 1960s. Today, the Burkard 7-Day volumetric trap and the Rotorod sampler are the most commonly used for research.
However, there are certain negative aspects to the spore traps above. Mainly, they are manually operated. Firstly, someone has to drive out to the trap to collect the slides daily or weekly. This can take a while, especially when traps are located in remote locations, such as rural Western Australia. Secondly, slides can be dropped or broken in transport in the back of a ute, rendering the data completely useless - unfortunate situations happen! Lastly, the results depend on the expertise of the person doing the analysis; this process requires an expert and is very time-consuming. The hours of driving and expert analysis can make this overall process slow and quite expensive. To add insult to injury, by the time slides are collected and analysed, it is often too late to even take action.
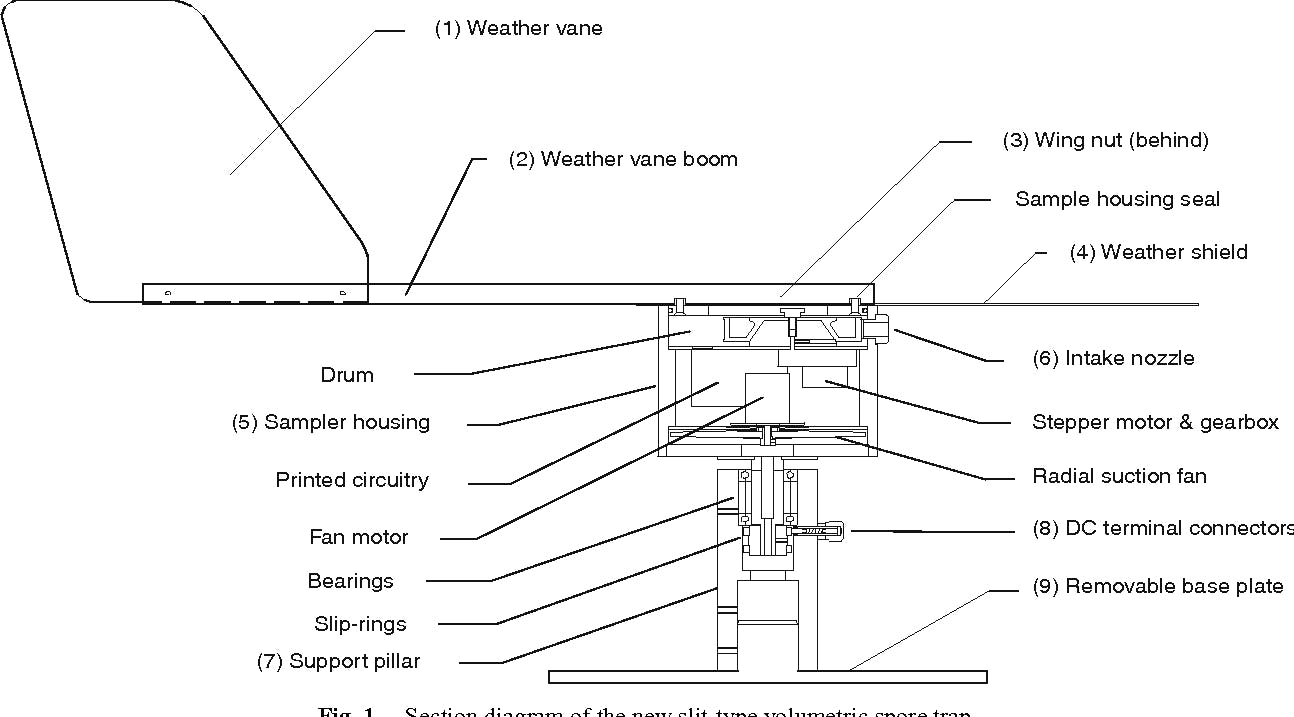
Section diagram of the slit-type volumetric spore trap. Source: Fig. 1. H. Neumeister-Kemp et al., 2011.
Next-gen Spore Trapping - BioScout
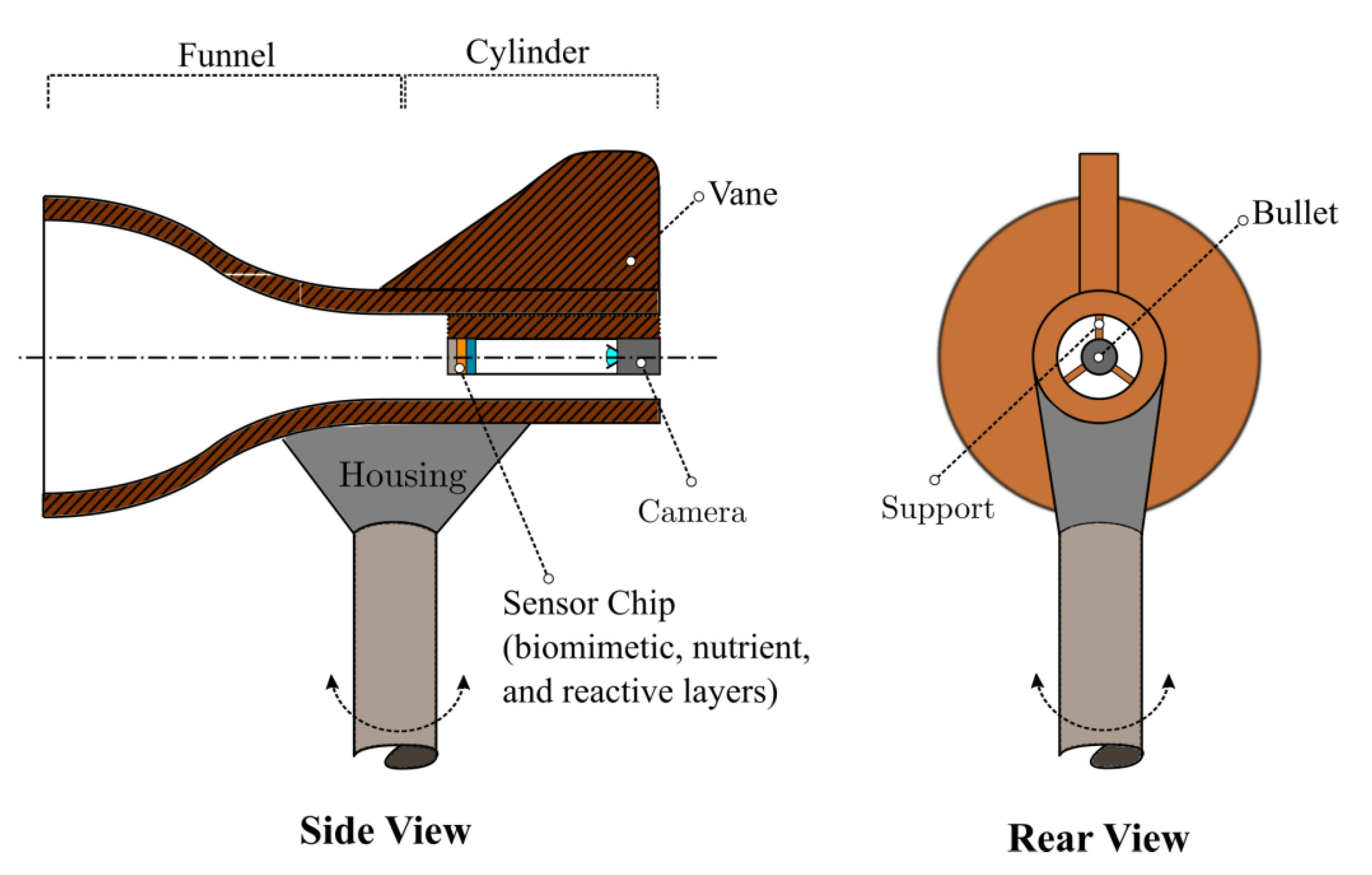
So, at BioScout, we have developed an autonomous spore sampler similar to the Burkard trap using patented technology and automated microscopy (see image 2 below). The data is uploaded to a cloud-based server which runs our machine learning algorithms to automatically identify and classify spores in real-time. This eliminates the need for all that labour, making spore trapping quick and efficient. This information is then provided to growers and interest groups fast enough and in a way that allows preventative action. Pretty neat, hey?! Time for me to get back to tracking airborne crop diseases!

Examples of spore traps. A. Passive deposition trap, B. Rotating arm impactor trap, C. Burkard 7-day recording volumetric sampler, D. Spornado passive funnel trap, and E. Burkard high-volume cyclone trap. Source: Fig. 1, Van der Heyden et al., 2021.
References
Lacey, M.E., West, J.S., 2007. The Air Spora: A manual for catching and identifying airborne biological particles. Springer Science & Business Media.
Van der Heyden, H., Dutilleul, P., Charron, J.-B., Bilodeau, G.J., Carisse, O., 2021. Monitoring airborne inoculum for improved plant disease management. A review. Agronomy for Sustainable Development 41, 1–23. https://doi.org/10.1007/s13593-021-00694-z